Our History
Our History
Ionis’ RNA-targeted technology translates genomic insights into potentially transformative therapeutics.1
Creators of Trailblazing Innovation
Creators of Trailblazing Innovation
Ionis created the foundational chemistry behind our RNA-targeted therapeutics and have invested time and resources toward further advancements of our platform technology.1-3
Advances that have come from our research include4-8:
Developing the chemistry that is used as the basis for commercialized RNA-targeted therapeutics
Optimizing administration techniques and creating chemical modifications designed to deliver RNA-targeted therapeutics to target tissues
Creating iterative screening and refining processes to identify RNA-targeted therapeutics for clinical settings
Pioneering mechanisms to modulate gene expression
The success of nusinersen, tofersen, and eplontersen – all Ionis-originated, FDA-approved medicines – are examples of our ability to potentially bring life-changing options to patients with neurologic diseases.1,9-12
Ionis continues to build upon its pioneering platform and foundational knowledge to develop therapeutics that can alter disease trajectory.1,7,9,13
The Timeline of Ionis Neurology1,4,10,11,14-24
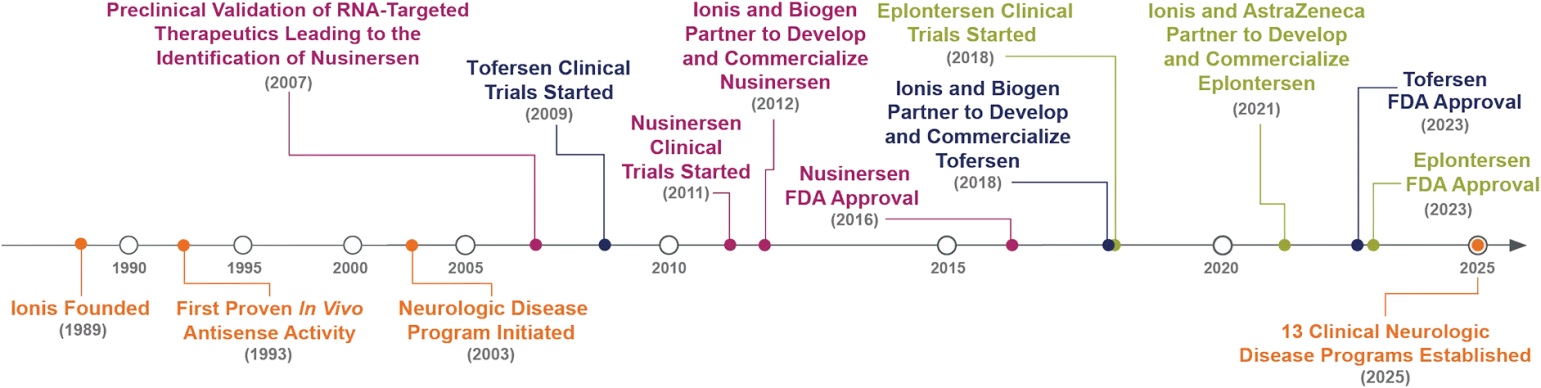
FDA, US Food and Drug Administration.
References
- Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20(6):427-453.
- Crooke ST. RNA-directed therapeutics at Ionis. Nature. 2019;574(7778):1-3.
- Bajan S, Hutvagner G. RNA-based therapeutics: from antisense oligonucleotides to miRNAs. Cells. 2020;9(1):137.
- Ionis Pharmaceuticals. The creation of RNA targeting technology. December 2019. Accessed March 3, 2025. https://ir.ionispharma.com/static-files/1cada39c-0fa4-4e90-afdf-e85fa258e03b/
- Crooke ST, Liang XH, Baker BF, Crooke RM. Antisense technology: a review. J Biol Chem. 2021;296:100416.
- Ionis Pharmaceuticals. Ionis Innovation Day. October 4, 2023. Accessed March 3, 2025. https://ir.ionis.com/static-files/8b71dc65-dad9-4368-9014-604c5b203ca1/
- Partridge W, Xia S, Kwoh TJ, Bhanot S, Geary RS, Baker BF. Improvements in the tolerability profile of 2'-O-methoxyethyl chimeric antisense oligonucleotides in parallel with advances in design, screening, and other methods. Nucleic Acid Ther. 2021;31(6):417-426.
- Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics [published correction appears in Cell Metab. 2019 Feb 5;29(2):501. doi: 10.1016/j.cmet.2019.01.001]. Cell Metab. 2018;27(4):714-739.
- Bennett CF, Kordasiewicz HB, Cleveland DW. Antisense drugs make sense for neurological diseases. Annu Rev Pharmacol Toxicol. 2021;61:831-852.
Qalsody. Package insert. Biogen MA Inc. Updated April 2023. Accessed July 15, 2025. https://www.biogencdn.com/us/pdfs/qalsody-prescribing-information.pdf
Wainua. Package insert. AstraZeneca. Updated September 2024. Accessed July 15, 2025. https://www.azpicentral.com/pi.html?product=wainua
- Ionis Pharmaceuticals. Medicines. Accessed July 15, 2025. https://www.ionis.com/medicines/
Ionis Pharmaceuticals. Corporate Responsibility Report 2023. Accessed July 15, 2025. https://www.ionis.com/sites/default/files/2024-08/Ionis-2023-Corporate-Responsibility-Report.pdf
- Bennett CF, Krainer AR, Cleveland DW. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci. 2019;42:385-406.
- Hua Y, Vickers TA, Baker BF, et al. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5(4):e73.
- Safety, tolerability, and activity study of ISIS SOD1Rx to treat familial amyotrophic lateral sclerosis (ALS) caused by SOD1 gene mutations (SOD-1). ClinicalTrials.gov identifier: NCT01041222. Updated April 13, 2012. Accessed July 15, 2025. https://www.clinicaltrials.gov/study/NCT01041222/
- An open-label safety, tolerability, and dose-range finding study of nusinersen (ISIS 396443) in participants with spinal muscular atrophy (SMA) (SMNRx). ClinicalTrials.gov identifier: NCT01494701. Updated February 18, 2021. Accessed March 4, 2025. https://www.clinicaltrials.gov/study/NCT01494701/
- Ionis Pharmaceuticals. Press release. January 4, 2012. Accessed February 4, 2025. https://ir.ionispharma.com/news-releases/news-release-details/biogen-idec-and-isis-pharmaceuticals-announce-collaboration/
- Biogen. Press release. December 6, 2018. Accessed February 5, 2025. https://investors.biogen.com/node/17506/pdf
- Evaluate the safety and tolerability, as well as the pharmacokinetic and pharmacodynamic profiles of single and multiple doses of eplontersen administered subcutaneously to healthy volunteers and patients with hereditary transthyretin-mediated amyloidosis (hATTR). ClinicalTrials.gov identifier: NCT03728634. Updated December 19, 2022. Accessed March 4, 2025. https://www.clinicaltrials.gov/study/NCT03728634/
- AstraZeneca. Press release. December 7, 2021. Accessed February 4, 2025. https://www.astrazeneca.com/media-centre/press-releases/2021/astrazeneca-ionis-to-collaborate-on-eplontersen.html#/
- Spinraza. Package insert. Biogen MA Inc. Updated April 2024. Accessed March 3, 2025. https://www.spinraza.com/content/dam/commercial/spinraza/caregiver/en_us/pdf/spinraza-prescribing-information.pdf
- Everett WH, Bucelli RC. Tofersen for SOD1 ALS. Neurodegener Dis Manag. 2024;14(5):149-160.
- Ionis Pharmaceuticals. Pipeline. Accessed March 4, 2025. https://www.ionis.com/science-and-innovation/pipeline/
